Thursday, 5 March 2026
FDA highlights associated risks of herbal medicine Kratom
Kratom contains alkaloids mitragynine and 7-hydroxymitragynine that have similar effects as opioids. The United States Food and Drugs Administration (FDA) has raised the issue about the potential safety risks associated…

Kratom contains alkaloids mitragynine and 7-hydroxymitragynine that have similar effects as opioids.
The United States Food and Drugs Administration (FDA) has raised the issue about the potential safety risks associated with the botanical substance, Kratom.
Kratom grows naturally in several Southeast Asian countries such as Thailand, Malaysia, Indonesia and Papua New Guinea. It has been used for centuries by local farmers and populations as part of several herbal remedies.
It contains alkaloids mitragynine and 7-hydroxymitragynine that have similar effects as opioids but do not affect breathing of the individual. Exact actions of the compounds present in this plant is not known.
Several online websites and shops sell this product in the US claiming that kratom can cure several mental health conditions such as depression, anxiety and pain and also be used as a de-addiction agent.
The FDA statement emphasizes that kratom naturally has similar effects like narcotics and opioids. This means that regular usage of these products can lead to addition and abuse problems. Severe over-dosage may also lead to death.
At present the FDA is taking steps to curb the use of kratom by preventing its shipments, conducting seizures, voluntary destruction of kratom containing products etc.
Technology
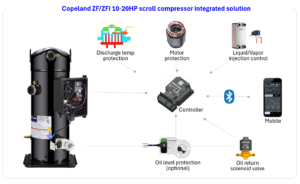
Copeland Launches ZF/ZFI 10-20HP Scroll Compressors
Mar 05, 2026 | Company News
WA Scientists Discover New Deep-Sea Crustacean Stocks with Strong Commercial Potential
Mar 05, 2026 | Australia
Food Testing
NSF and Circle H Collaborate to Enable Global Certification Access
Mar 04, 2026 | Company News
Australian Medical Bodies Push for Compulsory Health Star Labelling
Feb 24, 2026 | Australia
Tim Hortons Singapore Secures Majlis Ugama Islam Singapura Halal Certification Ahead of Ramadan
Feb 23, 2026 | Company News
More Popular
Copeland Launches ZF/ZFI 10-20HP Scroll Compressors
Mar 05, 2026 | Company News
Singapore Expands Support for Local Farms to Strengthen Food Security
Mar 05, 2026 | Food Security