Saturday, 28 February 2026
Delivery of probiotics efficiently to the intestine: the acid test
Introducing Bac-in-a-Box a new technology for the efficient protection and delivery of probiotic bacteria and yeast to the intestines of human and animals. The ability to deliver probiotics efficiently to…
Introducing Bac-in-a-Box a new technology for the efficient protection and delivery of probiotic bacteria and yeast to the intestines of human and animals.
The ability to deliver probiotics efficiently to the lower gastric tract would allow much lower doses of probiotics to be given and would increase their efficacy. This is because the acidic environment of the stomach has evolved to sterilize food and thus forms a tough barrier that has to be overcome if probiotics are to reach the intestine where they have their beneficial effects. The stomach is a highly effective barrier, killing almost all ingested bacteria. Although acid coatings have been developed for drugs, these are generally not compatible with the growth and survival of living organisms like probiotics. Moreover, studies have shown that extremely high numbers of at least one hundred million (108) viable probiotic bacteria must repeatedly reach the intestine for health benefits to be achieved for the patient (Govender et al., 2014, AAPS Pharm Sci Tech.15, 29-43 and references therein).
It is typical to lose 100 to 1,000 fold viability during storage of probiotics at room temperature. Indeed, Canada and Italy require a minimum dose of 10e9 viable cells measured as colony forming units (cfu) to be present in probiotic products until the end of the shelf life (Hill et al., 2014, Nat Rev Gastroenterol Hepatol 11, 506-514). In addition to this loss, another 1,000 to 10,000 fold viability is lost during passage through the stomach due to the action of the acidic environment as well as digestive enzymes. Thus ideally at least 1013 viable probiotic should be dosed per day to have a health benefit. Typical doses for over the counter probiotics rarely exceed 5×1010, so around a 1000 doses would need to be taken per day to achieve a therapeutic effect.
Moreover, the requirement for continued maintained therapeutic levels of probiotics requires regular probiotics consumption, as has been demonstrated in dose-response studies. In such studies, probiotics like Lactobacillus rhamnosus GG only transiently colonize the gastro-intestinal tract, for example fifteen days after terminating the administration of L. rhamnosus GG in adults, the probiotic bacterium could only be recovered from stool samples of 27% of the volunteers (Terpou et al., 2019, Nutrients 11, 1591).
A number of newer technologies are currently being developed to achieve better delivery efficiencies for probiotics so that more living bacteria and/or yeast can make it to the gastrointestinal tract. These include chitosan, alginate plus gelatinized starch and cellulose sulphate based encapsulation techniques. Austrianova has pioneered the use of cellulose sulphate for the acid protection of probiotics using its Bac-in-a-Boxâ technology. As well as protecting against stomach acid, Bac-in-a-Boxâ allows better long-term storage at ambient temperatures. Depending on the probiotic strain this can be as little as 10 fold loss over one year at room temperature.
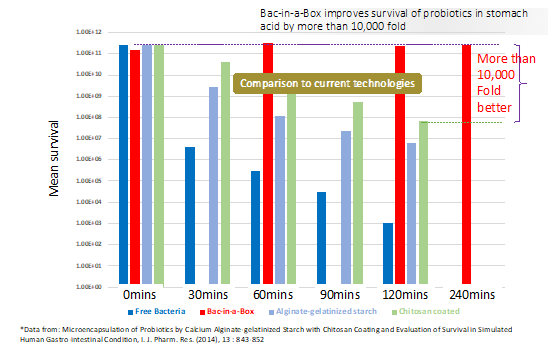
A comparison of the ability of chitosan, alginate plus gelatinized starch and cellulose sulphate to protect Lactobacillus casei bacteria from simulated human gastric fluid (pH2 and containing the digestive enzyme pepsin) is shown in Figure 1. Although all three technologies afforded protection compared to non-encapsulated bacteria (Zanjani et al., 2014, Ir J Pharmaceut Res 13, 843-852), Bac-in-a-Boxâ gave a 10,000 fold better protection than chitosan and a 100,000 fold better protection than alginate plus gelatinized starch after 3 hours exposure to simulated human gastric fluid. Moreover, addition of artificial bile for an additional hour after 4 hours incubation in simulated human gastric fluid does not appreciably affect viability of L. casei (not shown). The ability Bac-in-a-Boxâ to protect probiotics from the harsh, acidic conditions of the stomach also has been demonstrated for a variety of Lactobacillus species (including L. acidophilus, L. salivarius, L. plantarum, L. casei), Bifidobacterium (B. Lactis, B. longum), Lactococcus (L. lactis), Escherichia coli and the yeast Saccharomyces cerevisiae boulardii.
Low levels of cellulase are produced by commensal organisms in the gut of humans and other animals (although these levels are much higher in ungulates). It was shown that cellulase concentrations as low as 0.05 U/ml are sufficient to cause Bac-in-a-Boxâ capsule disruption. To demonstrate that Bac-in-a-Boxâ can protect probiotics in vivo, mice were administered encapsulated, labelled E. coli by gavage. Mice sacrificed 24 hours post-gavage showed the presence of the bacteria in the caecum and colon. Moreover, living labelled bacteria could be detected at significantly higher levels in fecal pellets for up to at least 24 hours post-ingestion, even though the transit time from mouth to anus in mice is 4-6 hours and suggesting that colonization of the intestines had occurred. Importantly, no obvious signs of toxicity were associated with Bac-in-a-Boxâ in these mouse (or indeed in other ongoing large animal) experiments.
In summary, Bac-in-a-Boxâ is at the forefront of new technologies for the efficient protection and delivery of probiotic bacteria and yeast to the intestines of human and animals. Moreover, cellulose sulphate is a natural and biodegradable biopolymer and cellulose is routinely used as a bulking agent in foods. Thus, Bac-in-a-Boxâ is a useful protective delivery technology for probiotics, both for use in humans as well as in ongoing farm animal studies.
Presented by;
Brian Salmons, Ph.D., Austrianova, Biopolis, Singapore
Biotech founder and industry expert in cell and microbiology
John A. Dangerfield, Ph.D., Austrianova, Biopolis, Singapore
Biotech expert, currently focusing on agritech and cosmeceuticals
Prof. Walter H. Gunzburg, Austrianova, Biopolis, Singapore
Professor of Virology at the University of Veterinary Medicine, Vienna, Austria and Biotech entrepreneur
Technology
Ingredion Thailand Achieves 100% Sustainably Sourced Cassava
Feb 27, 2026 | Company News
Deakin University and Bellarine Foods Partner to Develop Sustainable Marine-Derived Proteins
Feb 26, 2026 | Australia
Royal Unveils Refreshed Jute Bag Design for 20lb Authentic Basmati
Feb 25, 2026 | Company News
Food Testing
Australian Medical Bodies Push for Compulsory Health Star Labelling
Feb 24, 2026 | Australia
Tim Hortons Singapore Secures Majlis Ugama Islam Singapura Halal Certification Ahead of Ramadan
Feb 23, 2026 | Company News
More Popular
Fagron Acquires Pharmavit Europe for €68Mn to Expand Nutraceutical Portfolio
Feb 27, 2026 | Company News
Arla Foods Invests EUR 300Mn in New Cheese Dairy in Sweden
Feb 27, 2026 | Company News
Beyond Meat Broadens Portfolio Beyond Protein with Sparkling Plant-Based Drink Line
Feb 27, 2026 | Beverages






