Thursday, 5 March 2026
FSANZ requests feedback on allowing use of cultured quail as novel food
The application is from Vow Group Pty Ltd which is seeking approval to allow the use of cultured quail cells originating from quail as an ingredient in food products Food…

The application is from Vow Group Pty Ltd which is seeking approval to allow the use of cultured quail cells originating from quail as an ingredient in food products
Food Standards Australia New Zealand (FSANZ) has announced the initiation of the first public consultation round on an application seeking to amend the Australia New Zealand Food Standards Code (the Code). The amendment will allow the use of cultured quail cells as novel food.
This is the first cell-cultured food application to be assessed in Australia and New Zealand.
The application is from Vow Group Pty Ltd which is seeking approval to allow the use of cultured quail cells originating from quail as an ingredient in food products.
This first call for submissions seeks views on FSANZ’s risk assessment which focuses on the first three stages of cell-based food production – cell line, method of production and cell harvest.
The assessment found the cultured quail cell line is genetically stable and microbiological risks with cell line sourcing and harvest are very low.
FSANZ also found no toxicological, nutritional safety or food allergenicity concerns associated with the consumption of the cultured quail cells.
FSANZ looked at the labelling requirements for this product including undertaking research into consumer perceptions of cell-based food and associated terminology. The first call for submissions proposes the term ‘cell-cultured’ be used in the labelling of the product.
Technology
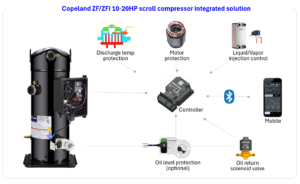
Copeland Launches ZF/ZFI 10-20HP Scroll Compressors
Mar 05, 2026 | Company News
WA Scientists Discover New Deep-Sea Crustacean Stocks with Strong Commercial Potential
Mar 05, 2026 | Australia
Food Testing
NSF and Circle H Collaborate to Enable Global Certification Access
Mar 04, 2026 | Company News
Australian Medical Bodies Push for Compulsory Health Star Labelling
Feb 24, 2026 | Australia
Tim Hortons Singapore Secures Majlis Ugama Islam Singapura Halal Certification Ahead of Ramadan
Feb 23, 2026 | Company News
More Popular
Copeland Launches ZF/ZFI 10-20HP Scroll Compressors
Mar 05, 2026 | Company News
Singapore Expands Support for Local Farms to Strengthen Food Security
Mar 05, 2026 | Food Security