Friday, 20 February 2026
Beckman Coulter Life Sciences launches BAT to improve allergy research
The new BAT test eliminates that risk, testing for multiple allergens at once through a blood draw- saving hours of food testing and exposure to potentially harmful reactions Beckman Coulter…
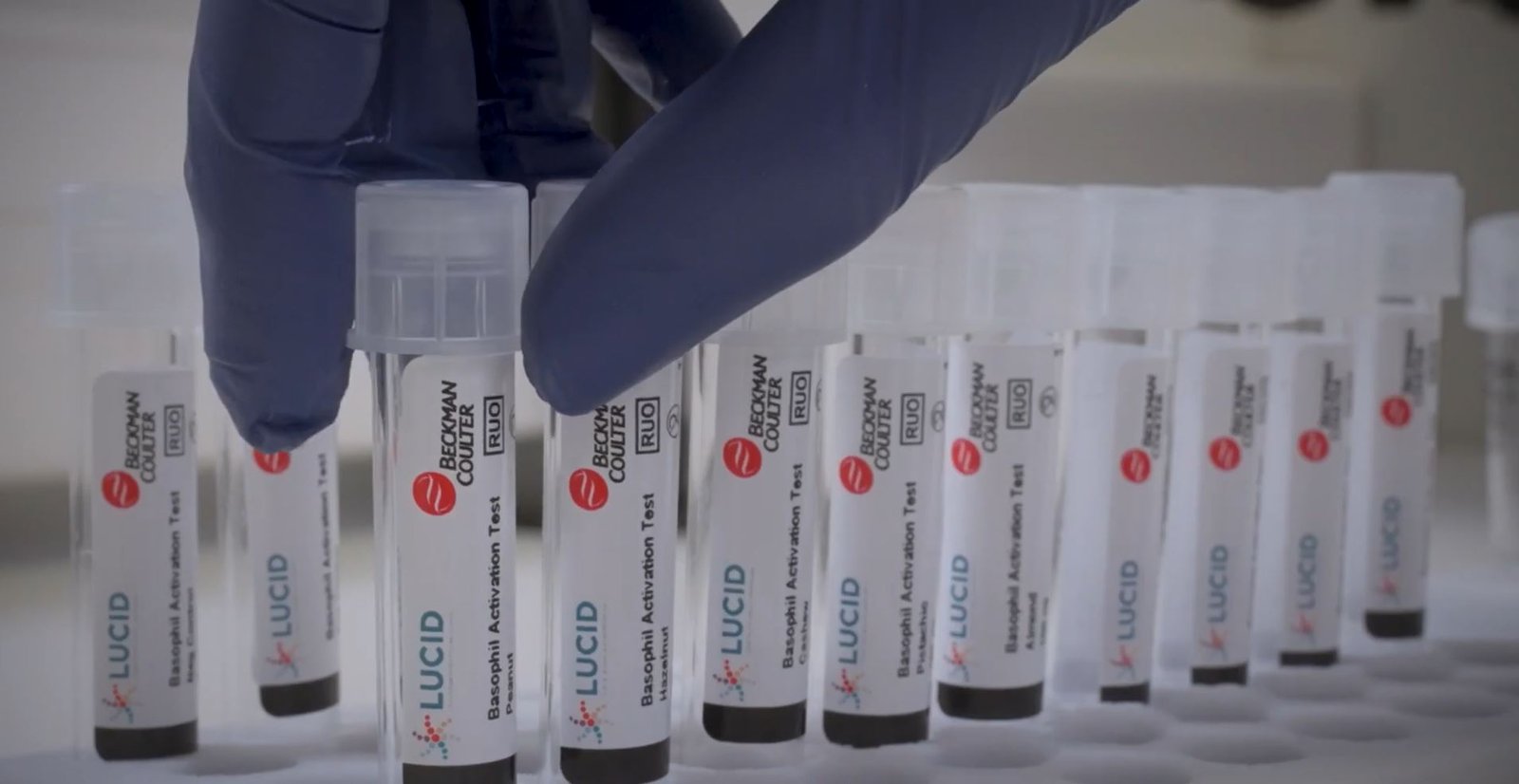
The new BAT test eliminates that risk, testing for multiple allergens at once through a blood draw- saving hours of food testing and exposure to potentially harmful reactions
Beckman Coulter Life Sciences, a global leader in laboratory automation and innovation, introduces the Next-Generation Basophil Activation Test (BAT) for research use only to more effectively characterize food allergies without exposure to potentially harmful allergens.
Approximately 220 million people globally suffer from at least one food allergy. Most food allergies and treatment efficacies are currently determined through a dated and rigorous Oral Food Challenge (OFC), exposing a patient to gradual amounts of foods to determine if they have a reaction, which could include anaphylaxis in some situations. The new BAT test eliminates that risk, testing for multiple allergens at once through a blood draw- saving hours of food testing and exposure to potentially harmful reactions.
“This offers a groundbreaking alternative in allergy testing, removing patient obstacles and reducing ethical concerns associated with direct allergen exposure in vulnerable participants,” said Jean-Marc Busnel, PhD, Principal Investigator and Senior Staff Research Scientist. “For too long, these concerns have limited critical advancements, putting people at risk of not having access to life-saving treatment options should an allergic reaction occur. By removing these barriers, this test unlocks an opportunity to finally expand food allergy drug development and research. This reinforces our mission at Beckman Coulter Life Sciences to deliver tools that empower clinicians and improve patient outcomes.”
The innovation follows the FARE (Food Allergy Research and Education) Innovation Award Diagnostic Challenge, which Beckman Coulter Life Sciences received in 2022. The $1 million award helps foster the development of improved testing methods in the field of food allergies.
“At FARE, we have prioritised supporting the development of safer alternatives to oral food challenges that can expose patients to risk and induce anxiety,” said Sung Poblete, PhD, RN, CEO of FARE. “The introduction of this next-generation Basophil Activation Test marks a significant advancement, moving diagnostic innovation from the bench toward future clinical application, paving the way for safer, more accessible food allergy research while offering the opportunity to stimulate progress and we are excited about the promise it holds for research and development.”
Technology
Tetra Pak extends paper-based barrier packaging to high-speed packaging lines in Asia
Feb 19, 2026 | Company News
Food Testing
Redefining Trust in Organic Foods through Independent Testing
Feb 13, 2026 | Food Safety and Testing
AFNOR International Eyes Global Food Safety Growth with HACCP Group Takeover
Feb 04, 2026 | Australia
More Popular
Strong Q4 Drives Coca-Cola Consolidated to Record FY2025 Results
Feb 20, 2026 | Beverages
The Kraft Heinz Company Appoints Nicolas Amaya as President, North America
Feb 20, 2026 | Company News
Orkla Food Ingredients acquires Senna
Feb 20, 2026 | Company News






