Sunday, 8 March 2026
DNH receives reapproval for Xylitol health claim in Korea
Ministry of Food and Drug Safety concludes that daily consumption of 5-10 grams of XIVIA® xylitol helps reduce risk of dental caries occurrence The Korea Ministry of Food and Drug…

Ministry of Food and Drug Safety concludes that daily consumption of 5-10 grams of XIVIA® xylitol helps reduce risk of dental caries occurrence
The Korea Ministry of Food and Drug Safety (MFDS) recently re-approved the health claim that XIVIA® Xylitol, part of the DuPont™ Danisco® range of ingredients, reduces the risk of dental caries occurrence, in consumers from 3 to 80 years old.
In Korea, functional ingredients that have passed 10 years since a health claim approval must undergo mandatory re-evaluation. With the new re-approval, DuPont Nutrition & Health can continue to work with manufacturers to create sugar-free products that improve oral health.
XIVIA® Xylitol, a naturally occurring wood-based sweetener, delivers full functionality of taste and sweetness at 50% of the calorie level of sugar. In the re-evaluation, MFDS reviewed 146 research reports, including 94 clinical trials, and concluded that XIVIA® helps reduce risk of caries occurrence with an adjusted effective daily dosage from 10-25 grams to 5-10 grams, similar to international dental association standards.
In 2016, the South Korean government introduced a legislation that requires food manufacturers to provide more information on sugar levels in their products.With consumers’ rising health consciousness in Korea, many local companies are already engaged in efforts to reduce sugar content in their food and beverage products.
The use of XIVIA® Xylitol will help manufacturers address dental caries demands and create safe sugar substitute products in many applications such as dairy, beverages, bakery, confectionery, dietary supplements and more.
Technology
Sidel introduces SWING Evo Pasteuriser, Redefines Efficiency through Intelligent Design
Mar 06, 2026 | Company News
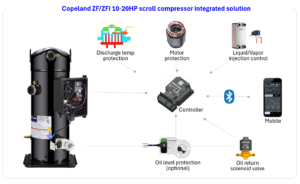
Copeland Launches ZF/ZFI 10-20HP Scroll Compressors
Mar 05, 2026 | Company News
Food Testing
NSF and Circle H Collaborate to Enable Global Certification Access
Mar 04, 2026 | Company News
Australian Medical Bodies Push for Compulsory Health Star Labelling
Feb 24, 2026 | Australia
Tim Hortons Singapore Secures Majlis Ugama Islam Singapura Halal Certification Ahead of Ramadan
Feb 23, 2026 | Company News
More Popular
Nutrition Education Drives Adoption of Seaweed and Mussels among Bangladesh Coastal Communities
Mar 06, 2026 | Food Security
Sidel introduces SWING Evo Pasteuriser, Redefines Efficiency through Intelligent Design
Mar 06, 2026 | Company News